Figure 1: The shell of the Magician's cone (Conus magus)
© InDepth Images Kwajalein Photo credit: Jeanette Johnson. Retrieved from http://www.underwaterkwaj.com/shell/cone/Conus-magus.htm
Table of Contents
1. Introduction
The Magician’s cone (Conus magus) is a type of cone snail belonging to the Family Conidae. The genus Conus is quite large, consisting of over 803[1] species all of which are thought to be carnivorous[2] . They are widely distributed in the tropical Pacific and Indian Oceans, most commonly occurring in coral reefs. They live in both tropical and temperate water, occurring in both intertidal regions to very deep waters of almost 1000m[3] .
The Magician’s cone is a piscivorous (fish-eating) animal, relying primarily on using its venom
to disable its prey. The Magician's cone is one of the 23 species of cone snails that have been reported to occur in Singapore, though it is a rare resident[4] . In fact all cone snail species that have been recorded in Singapore are rare residents that have been described to occur sporadically in the offshore coral reefs around Singapore such as Cyrene reef[5] and Lazarus Island coral reef[6] [7] . With the increasing damage to our coral reefs and the loss of the cone snails’ habitat, it has now become extremely rare to find a live cone snail in Singaporean waters.
2. External Morphology
2.1. Visible animal
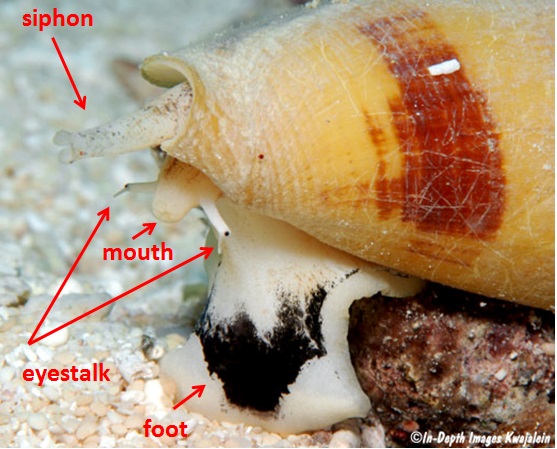
Figure 2: The above features are visible when the animal is extended out of its shell
© InDepth Images Kwajalein Photo credit: Jeanette Johnson. Retrieved from http://www.underwaterkwaj.com/shell/cone/Conus-magus.htm
2.2. Shell structure
The Magician's cone has a medium-sized shell which is ventricosely conical in shape with a subangulate shoulder. It has low spire which is concave with obvious sutures. The teleconch sutural ramps are slightly concave and spirally ribbed with widely spaced, black-brown radial markings. The sculpture has three spiral ads and fine axial grooves with several spiral ribs at the base of the body whorl. The aperture is white and widens anteriorly, with a smooth outer lip. The average size of this shell ranges from between 55-70mm though large specimens as long as 89mm have also been observed. The colour is generally whitish with a brown axial blotches arranged into two spiral bands on the body whorl though there is a wide-spectrum of intra-specific variation in this species as shown in Figure 3[8] [9] .
Figure 3: Intraspecific variation in conus magus shells.
Photo credit: Richard Parker Retrieved from https://commons.wikimedia.org/wiki/Category:Conus_magus#/media/File:Conus_magus_dorsum_001.jpg
4.Predation in cone snails
While all cone snails are predatorial species, different species vary on the specificity of their diet. Generally, species of cone snails that appear singularly tend to be more generalist feeders, whereas cone snails species that occur alongside each tend to be more specialist feeders, which each species occupying its own individual ecological niche with a fixed set of food preferences. As many as 20 species of cone snails may occur in the same reef, which is an indication of how specialised the diet of different cone snail species can be[10] [2] .All cone snails are carnivorous though and can generally be split up into three groups based on preferred prey species. Most of the cone snail species are vermivorous meaning they primarily feed on worms species such as polychetes. While not as common as vermivores, there are also some species of cone snailst that are Molluscivorous and mainly prey on gastropods. Lastly, the least common group of cone snails are the piscivorous species that feed on fishes and which have smaller populations than the known molluscivorous or vermivorous species.[2]
4.1Morphological adaptations for predation
i) Prey Detection
All Cone snails possess an osphradium , an organ that houses chemo-receptors that are used for prey detection. Some species have been observed to even “track” their prey with their chemo-receptors, moving about with their proboscis extended in order to indentify the position of the prey.[2]
ii) Venom injection
The main morphological adaptation that cone snails possess to enable their predatory carnivorous diet is the development of a cocktail of conotoxins that can form a potent venom that can effectively paralyse and disable their prey.
Cone snails possess a proboscis which facilitate the injection of venom into the prey. The venom is secreted and stored in a venom gland that opens up into the lumen of the proboscis. The distal end of the venom gland has a muscular bulb that upon contraction will supply the force necessary to inject the venom into the prey. The radular teeth are elongated and pointed and are positioned at the base of the proboscis. A single radular tooh will be used as a “harpoon” of sorts that will penetrate the prey when the cone snail strikes. The tooth is hollow and is connected to the lumen of the proboscis that is filled with venom. Upon contact with the prey, the radular tooth will the inject the venom into the prey’s body. The tooth itself is barbed, ensuring that it will be securely lodged to the prey’s body and that the prey remains attached to the snail’s proboscis. The prey may struggle immediately after the injection of the venom but will soon be paralysed and immobile, after which the cone snail begins to feed. Each radular tooth is only used once per attack. If the cone snail is successful in striking its prey, the tooth will be swallowed along with its prey. If the strike is unsuccessful , the tooth is discarded and new radular tooth is positioned for the next strike.
[2]
iii) Prey Ingestion/ Digestion
Cone snails are able to extend and the increase the size of their mouth from anywhere between a few millimetres to a few centimetres, depending on the type of prey they are specialised for. Some species of cone snails, especially the piscivorous species, are adapted to be capable of swallowing prey twice their size. For piscivorous species, the fish prey may be as long as the snail itself and hence it cannot consume the entire length of the prey, both due to the prey size, and the size and shape of the shell limiting the amount of prey that can enter the alimentary canal of the snail at any one point in time. Instead the fish is digested bit by bit while in eusophagus, with digestive enzymes moving upwards from the gut to digest the prey. [2]
4.2. Piscivorous Cone Snails
The Magician’s cone is a piscivorous species, which generally possess more potent venoms than the non-piscivorous cone snail species.[2]The Magician’s Cone possess a particularly lethal concoction of conotoxins that are potent enough to even kill humans.
Piscivorous tend to be nocturnal as they typically hunt when their fish prey are inactive and are resting near the bottom of the ocean floor. Many species rely on ambush predation, hiding themselves almost completely within the sandy substrate on the ocean floor, leaving only their siphon sticking out, and striking when a prey swims near. [2]
There are three known methods through which piscivorous cone snails hunt their prey.
4.3. Hunting Techniques exhibited by Piscivorous cone snails
i) Taser-and-tether strategy/ Hook-and-line strategy
Cone snails employing this technique use their chemo-receptors to detect the presence of a fish prey. Olivera et.al (2015) has recently renamed this hunting strategy as the ‘Taser-and-tether strategy’, in reference to the fact that conotoxins in the Cone snail’s venom create strong electrical impulses in fish preys’ nervous systems such that it paralyses them, similar to the effect of a taser.Upon identifying the position of its prey, they extend their rostrum, a funnel-like structure formed by the muscular walls of the proboscis sheaths, and from the rostrum, the proboscis extends outwards almost like a fishing-line near where the fish is. Once the proboscis is close enough, the cone snail strikes, releasing its radular tooth to penetrate the prey, upon which the fish is envenomated. While the fish will struggle for a short duration, it will soon become immobile and stiff. The venom of cone snails that hunt with this technique is particularly specialised to ensure that the prey stiffens, as only if they prey is stiff can the cone snail successfully use its proboscis to pull the prey towards its mouth. Just before consumption, delayed-activation conotoxins within the cone snail’s venom will take it effect, making the dead prey’s body flaccid and thus easy to ingest. A couple of hours after ingestion, the cone snail will usually expel the scales and bones of the prey, as well as the radular tooth.
This is the technique employed by the Magician’s cone (Conus magus)[11] .
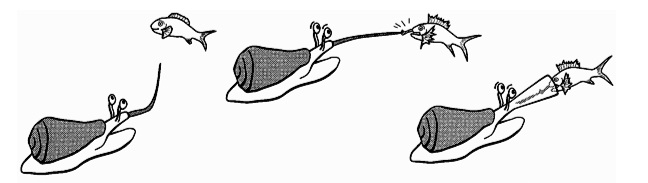
Figure 4: Diagram depicting the hook-and-line predation technique employed by piscivorous cone snails.
https://www.youtube.com/watch?v=JjHMGSI_h0Q
The video above shows a cone snail attacking its prey using the hook-and-line technique.
ii) Net-hunters/ engulfment strategy
These cone snails are able to enlarge and extend their rostrum outwards until it forms a “net” of sorts that engulfmultiple fishes at one time. In some species, the mouths of these cone snails are adapted to feature some finger-like projections that are reminiscent of the tentacles of sea anemone, to trick fishes into thinking that they are shelter.
Once the fishes are within its mouth, the snail will then use its radular tooth and “harpoon” its prey to paralyse them. The venom of net-hunter cone snails are specialised to have conotoxins that only induce flaccid paralysis, making the prey easy to consume. After a couple of hours, these cone snail species also expel the scales and bones of the prey, as well as the radular tooth.
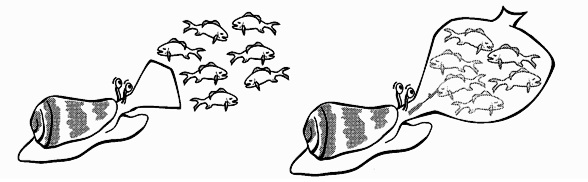
Figure 5: Diagram depicting the net-engulphment technique employed by piscivorous cone snails.
https://www.youtube.com/watch?v=UHiGuquJmpE
The video shows the Net-hunter technique employed by the Cone Snail species Conus tulipa to capture its prey.
Weaponizing insulin
Two species of cone snails ( Conus geographus and Conus tulipa) have been discovered to be able to use insulin as a biological weapon when hunting prey. They produce a modified and shortened version of insulin within their venom glands, that is distinct from the molluscan insulin that they use to regulate their own blood sugar. Interestingly, this modified insulin resembles the vertebrae insulin molecule found within fishes, but is shorter in length and seemingly faster in activation than regular verterbrae insulin. The cone snails release this modified insulin along with other toxins from their venom gland directly into the water around them, in what has been described as the ‘nirvana cabal’ of all venoms. The name is derived from the effect of the insulin on the prey, which enters nearby fishes’ bloodstream through their gills and body and plunges them into hypoglycaemic shock, disorientating them and effectively immobilising them.
iii) Strike and stalk strategy
This strategy has only been observed in Conus flavus. When the snail detects fish prey in vicinity, it will extend its striped proboscis towards the prey, much like the first stage of the taser-and-tether/hook-and-line strategy. Once the prey is within striking distance, the snail allows its radular tooth to penetrate the prey’s body, envenomating it. However, instead of tethering the prey and pulling it towards itself, Conus flavus allows the prey to swim away until it loses mobility due to being paralysed from the conotoxins within the injected venom. The cone snail then follows the prey until it is immobilised, before it proceeds to consume the immobile prey, tail-end first.
5.Venomology
5.1. Introduction to conopeptides
Cone snails are slow-moving predators and hence rely on the efficacy of their venom to hunt and capture their relatively fast-moving prey, especially in the case of piscivorous species.[12] The venoms are extremely complex, consisting over thousands of different conopeptides across all cone snail species.Thus far, over 3000 different conotoxins (peptides) have been identified and studied from the cone snail venoms since the 1970s. However, due to sheer volume and diversity of species in cone snails, it is estimated that there are over 41 million venom peptides expressed across all the different cone snail venoms[13] , making cone snail venom a huge resource for pharmacological study, with Wang and Chi (2004) describing cone snail venoms as “pharmacological treasures”[14] .
Most of conopeptides tend to be disulfide-rich molecules which are generally termed as ‘conoxtoinxs’.
Conotoxins tend to be very short peptides about 10-35 amino acids long and their most unique property is the extent of selectivity they display in having very specific target-receptors. Most conoxtoxins tend to act on volatage and ligand gated, potassium, calcium and sodium channels, as well as nicotic acetylcholine receptors and noradrenaline transporters. This makes them particularly suitable for pharmacological application and medical research as the incredible specificity conotoxins display can be used to isolate peptides that can be used to manufacture drugs to treat different diseases and symptoms.
Each individual conotoxin contains a signal sequence, followed by a pro region, and a mature peptide at the C-terminus. Most of the conotoxins studied thus far have been classified into venom gene superfamilies based on determining the identity of their signal sequence, as it has been found that the signal sequence of conotoxins tend to be highly conserved within different gene superfamilies.
Each individual cone snail species has its own distinct cocktail of conopeptides which is uniquely adapted to target a specific type of prey, which is a testament to great specificity of the diet in cone snails even among closely related species, which allows for multiple species of cone snails to occupy the same habitat as each individual species occupies its own ecological niche.
Therefore, since each cone snails species produces its own distinct venom cocktail, the combination of venom production genes found in any cone snail would be unique to that particularly species, such that variation can be clearly observed even among closely-related species. It has been found that venom peptide encoding genes in cone snail species exhibit an extremely accelerated rate of evolution through a mechanism known as a ‘focal hypemutation’ which could explain why the properties of the venom cocktails are highly different even for closely related species. This means, that studying venom encoding genes might provide a way to delimit cone snail species and classify them phylogenetically. The highly conserved regions in the venom peptides such as the signal sequences provide a way to do classification at the superfamily level. There are seven such superfamilies labelled ‘L’, ‘J’ , ‘P’ ,’ O’, ‘M’ and ‘T.’ Conus magus is classified under the ‘O’ superfamily with omega conotoxins.
5.2. Venom mechanism for piscivorous cone snails using the taser-and-tether/hook-and-line strategy
The venom cocktail of cone snails employing this strategy has two distinctive physiological impacts.The first impact is described as the ‘lightning-strike cabal’ where a specific group of conopeptides induces a large-scale depolarization of the axons located at the site of injection. The conopetides also “inhibit the desensitization of postsynaptic receptors in peripheral sensory circuitry” which triggers an “electrical storm” in the nervous system of the fish prey and effectively paralyses within seconds.
The second impact is described as a ‘motor cabal’ in which a second group of conopeptides get disseminated throughout the body of the fish prey through its circulatory system. These conopeptides inhibit neuromuscular transmission, effectively paralysing the skeletal musculature.
5.3. Evolution of venom and venom apparatus in piscivorous cone snails
Venom composition of piscivorous cone snails
As mentioned earlier, conopeptides are classified into gene superfamilies based on the conserved signal regions. It has been found that all piscivorous cone snails highly express a select few gene superfamilies, namely the A, M and O1 superfamilies. The A gene supefamily produces conopeptides such as α-GI which targets nicotinic acetylcholine receptors. The M gene superfamily produces conopeptides such as μ-GIIIA which targets the voltage-gated sodium channels. The O1 gene superfamily produces conopeptides such as ω-GVIA which targets voltage-gated calcium channels. All of these gene superfamilies produce conopeptides which are involved in inducing ‘motor cabal’ in the fish prey and consequently paralysing the fish’s skeletal musculature. This is an important part of the taser-and-tether/hook-and-line fish hunting strategy, as by paralysing the skeletal musculature of the fish prey, the fish’s body becomes stiff, allowing for the cone snail to reel the fish close to its mouth so that it can consume it.Radular tooth morphology of piscivorous cone snails
It has been found that the radular tooth morphology varies among piscivorous cone snails depending on the hunting technique they employ.i) taser-and-tether/hook-and-line strategy
Cone snails like Conus magus which use the taser-and-tether/hook-and-line strategy tend to have “strong radular teeth with a long accessory process” and often also possess an “additional bard that can tether fish securely” after the cone snail has successfully striked at its prey.
ii) Net-engulphment strategy
Cone snails employing this strategy such as Conus tulipa and Conus geographus are described to have “needle-shaped harpoon with a narrow base” which are “weakly barbed” at their tip.
Evolution of piscivorous cone snails- from a vermivourous ancestor?
i) Development of ‘lightning cabal’ conotoxinsIt has been hypothesised that the piscivorous cone snails have evolved from a vermivorous ancestor. Molecular data has suggested that the vermivorous ancestor possessed adaptations to fight off fish competitors for worm prey. This is thought to have eventually given rise to piscivory when the vermivorous ancestor eventually developed a venom component that caused pain in the fish competitors by activating the volatage-gated sodium channels in their nervous systems. This venom component was δ-conotoxin and is highly conserved among all piscivorous cone snails today and is also present in some vermivorous and molluscivorous species.
Subsequently along the evolutionary trajectory, it is thought that the ancestor developed the ability to produce another conotoxin that blocked the voltage-gated potassium channels in the nervous system of fishes. That combined with the effect of the δ-conotoxin created the ‘lighting cabal’ effect in the nervous systems of fish, causing a mass depolarisation of the axons localised at the site of the injection and triggering paralysis within a few seconds. With the fish now being effectively paralysed upon the injection of the venom, they are no longer food competitors and could have then be regarded as a possible prey item by the vermivorous ancestor. This could have then lead to opportunistic feeding of the paralysed fish prey by the vermivorous ancestor which in turn would likely created selected pressure to induce adaptations that would help improve the fish-prey capture ability of the vermivorous ancestor.
ii) Development of taser-and-tether/hook-and-line strategy
Over time, the ancestor would have developed the best combination of conopeptides within its venom to immobilise the fish prey. Subsequently, to further improve on the efficacy of the venom on the fish prey, it is likely that the ancestor would have developed radular tooth adaptations such as the development of a barbed accessory that would help the cone snail to more securely tether the prey, so as to ensure that there is proper injection of the venom into the fish prey and to limit the chances of the fish escaping before effective envenomation occurs.
Finally, the ancestor species would have become such an effective predator of fishes that it would have specialised in a piscivorous diet, which would have likely given rise to the various piscivorous cone snail species we see today.
iii) How many times did piscivory occur in cone snails?
Analysis of molecular data has suggested that piscivory is likely to have developed twice across different cone snail lineages. Piscivory is thought to have occurred separately in the in the subgenus Chelyconus which contains Atlantic cone snail species, while a different event is likely to have caused piscivory to evolve in the seven clades of piscivorous cone snails found in Indo-pacific. These seven clades are Asperella, Gastridium, Afonsoconus, Textillia, Pinonoconus, Embrikena and Phasmoconus.
While the two different piscivorous lineages share some similarities in terms of the hunting behaviour they employ as well as certain radular tooth adaptations such as the “presence of a strongly barbed accessory, the venom components they employ to cause ‘lightning cabal’ and ‘motor cabal’ are vastly different, with only the δ-conotoxins conserved between both lineages. The fact that the two lineages of piscivorous cone snails employ vastly different conopeptide combinations to achieve the same effect of paralysis and skeletal musculature immobilisation lends support to the hypothesis that piscivory evolved separately among the two lineages.
5.4. Properties of Conus magus venom
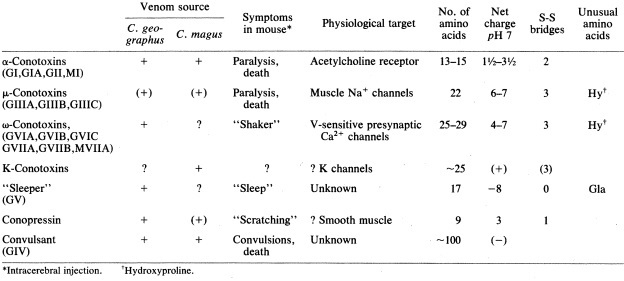
Table 1: The above table is a reproduction of the one published in Olivera et. al (1985) describing the physiological effects of different conopeptides isolated from Conus magus and Conus geographus on mice.The above figure is from a 1985 study by Olivera et.al examining the effects of different conotoxin components isolated from both Conus magus and another snail species Conus geographus on mice. As shown above, each conotoxin isolated has a very specific target receptor and precise effect.
For Conus magus, the most well characterised conotoxin among the ω conotoxins is the MVIIA conotoxin which very specifically targets only N-type Ca2+ receptors without affecting the other Ca2+ receptor types.
As shown above, it was found that when MVIIA was injected into mice intracerebrally (into the brain tissue), it induced tremors. However, when the same compound was later injected into mice intraperitoneally ( into abdominal cavity), there were no effects registered. This highlights the degree of specificity of the compound as it could only affect the N-type Ca2+ receptors that were present in the brains and had no effects on the other calcium channels located in the abdominal cavity.
However, when the MVIIA conotoxin was injected into the abdominal cavity of fish, the actual intended prey for Conus magus, it was found that it induced death in all the fishes tested which highlights efficacy of the Conus magus venom for its intended prey.[15]
5.5. Prialt
The MVIIA conotoxin is perhaps the most famous of all conotoxins disovered thus far, as it has been used to produce the drug Ziconotide used for managing neuropathic pain associated with malignant diseases like cancer, as well as other diseases like AIDS.[16] It is thought to be ten times more effective than opiods like morphine with none of the addictive properties making a particularly effective pain management drug.Ziconotide is marketed under the brand name Prialt and is the only FDA-approved conotoxin-based drug thus far.[17] [18]
5.6 Bioprospecting of Cone snail venom
Bioprospecting is defined as exploring our natural biodiversity to general valuable genetic or biochemical resources that can be applied in either scientific and/or commercial uses by Arico & Salpin (2005).With an estimated 41 million conopeptides across all the hundreds of species of cone snails, Cone snails are one of the richest natural resource for pharmacological research. Many other conotoxins from different cone snail species are currently undergoing clinical trials for different types of drug therapy as shown in the table below.
Table 2: The above table describes a few examples of different contoxins from different cone snail species currently undergoing clinical trials to be use in the manufacturing of drugs.
| Species |
Conopeptide |
Conotoxin class |
Target Receptor |
Intended treatment |
Clinical Trials |
| Conus purpurascens |
PVIIA |
k-conotoxin |
potassium channels in the heart |
Cardioprotection |
pre-clinical testing |
| Conus regius |
RgIA |
α-conotoxin |
Nicotinic Acetylcholine receptors |
Neuropathic pain |
Phase 1 |
There are many more other clinical trials currently undergoing involving conopetides isolated from the venom of several other cone snail species such Conus catus, Conus marmoreus, Conus victoriae, Conus geographus and Conus tulipa among many others.[19]
3.Life Cycle and Reproductive Biology
There appears to be limited information on the life cycle and reproductive process of the Magician’s cone and many other cone snail species in general. This could be attributed to the difficulty in keeping cone snails in captivity as well to study them in the wild for extended periods. In past studies, female cone snails have been typically observed to spawn only once per reproductive season. The only exceptions appear in unnatural laboratory conditions where due to the excess of food, female individuals have been observed to spawn multiple times within one reproductive season.Despite the lack of knowledge on the life histories of most cone snail species, a detailed study by Kohn & Perron (1994) has suggested that egg size is the key factor that influences the understanding of “reproductive energetics”, “temporal patterns of embryonic development and larval biology” and “dispersal potential”.[20]
Cone snail eggs are typically spherical and are contained within specialised structures known as egg capsules. These egg capsules are described to be shaped like a “flattened vase, with convex edges, a very short broad stalk attaching it to an adhesive basal plate and a translucent, apical exit window through which the larvae emerge at hatching” according to Kohn and Perron (1994). The capsules are made out of three components: ova, capsule wall material and intracapsular fluid. The egg capsules are known to be energy rich and it is thought that by conducting a calorie analysis of the egg mass, the reproductive effort exhibited by the individual female snail can be calculated.
3.1 Types of egg masses
Type I: Each capsule is directly attached to a hard substratum, the egg mass forming many short rows of a few capsules each.Type II: Only a few capsules are directly attached to a hard substratum. The rest of the capsules are affixed to the few capsules attached to the substratum, forming a tight cluster.
Type III: The first few capsules are deposited within a sand substratum , forming an anchor of sorts within the sedimentation.These buried capsules do not have eggs. The rest of the egg-bearing capsules are attached to these ‘anchor’ capsules within the sand substratum.
The capsules of the Type II and Type III egg masses are more loosely attached to the egg mass and hence can be easily separated and set adrift, in a form of dispersal.The Type II egg capsules are specifically adapted for this mode of dispersal. The egg capsules have a thicker and tougher wall material. Thus, if a Type II egg mass gets dislodged from the substratum and some of its egg capsules get filled air due to being punctured, the entire mass can simply float away following the flow of the ocean currents, and hence travel wide distances.
Egg mass type for the Magician’s Cone (Conus magus)
The Magician’s cone typically has egg masses of type I.
3.2 Embryonic development of Cone Snail Larvae
Based on an earlier study by Kohn et.al (1961) on 9 Cone snail species, there are about 8 described embryonic stages that occur after fertilisation.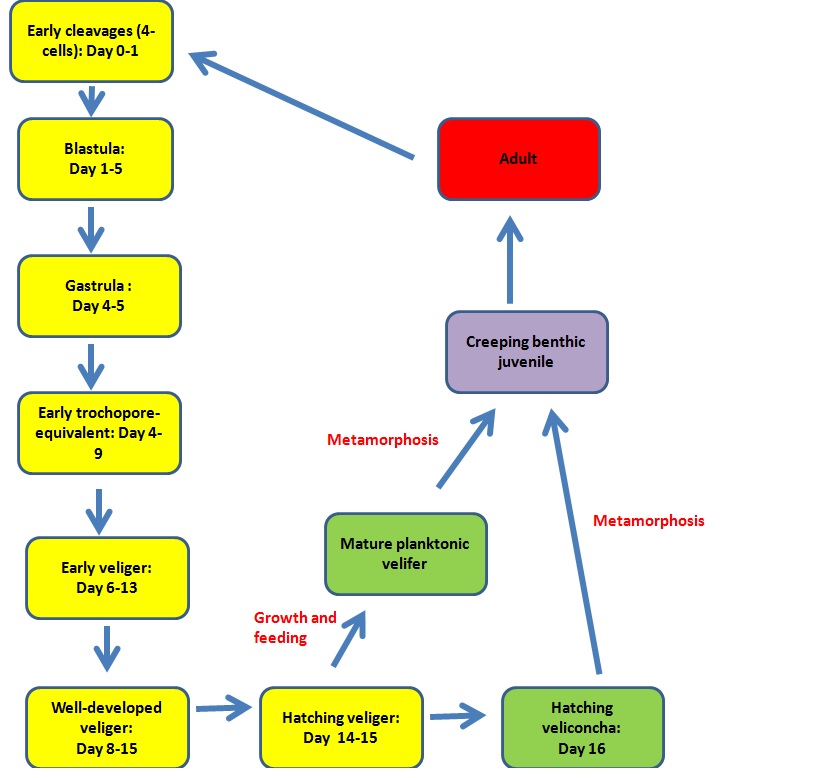
Figure 6: The above figure depicts life cycle that cone snail species undergo.
For most species, hatching occurs in the veliger phase. The larvae take on a planktonic state and are dispersed by ocean currents before they eventually metamorphosise into a benthic,creeping juvenile that eventually grows into an adult.The planktonic period is obligate for the larvae of these species, where they need to feed and grow rapidly, attaining some physiological and morphological changes before they are able to undergo metamorphosis.
In a few species like Conus magus, hatching occurs at the veliconcha stage and the planktonic stage is usually absent. They are typically lecithotrophic, meaning they do not require food and can depend on the energy supplied for the egg yolk for development. However, the larvae of some species are known to feed on unicellular algae if available. These larvae metemorphasise shortly after hatching, unlike the veliger larvae that undergo a long developmental period before attaining metamorphosis.
3.3 Relationship between egg sizes and developmental trajectories of Cone snail species
The most variable factor in cone snail reproductive biology is thought to be the size of the eggs, which can vary from anywhere between 125 μm to 1000 μm across the 800+ species of currently known cone snails.Hypothesis:
It is hypothesised that the size of the egg is directly related to the advancement of the cone snail species along its developmental trajectory, with larger eggs producing larvae that are more developed and more ready for metamorphosis, unlike smaller eggs, which tends to produce larvae that are less developed, and hence have a longer growing phase before they attain metamorphosis. The overall developmental trajectory is thought to be similar for all cone snail species.
Larger egg sizes (>480 μm) tend to have longer pre-hatching durations, with larvae hatched at the veliconcha stage. They are typically lecithotrophic being able to metemorphasise shortly after hatching without needing food.
Smaller egg sizes have shorter pre-hatching durations, with the larvae typically hatching at the veliger stage, with the larvae taking a planktonic state. Metemorphasis occurs after a much longer period, where the planktonic larvae slowly morphs into a creeping, benthic juvenile that becomes an adult. The planktonic period is obligate for the larvae of these species, where they need to feed and grow rapidly, attaining some physiological and morphological changes before they are able to undergo metamorphosis.
Veliger hatching state vs Veliconcha hatching state: Velum development
The most distinguishing characteristics that separates the different larval development strategies employed by different cone snail species appears to be the development of the velum which in turn seems to be dependent on the size of the egg. The velum is the organ that larval cone snails use for swimming and feeding and the larvae of various cone snails species have different velum growth and development pattern. It is thought that species with more well-developed velums are capable of feeding and growing more efficiently.
i) Veligers with small hatching sizes
Small velar lobes emerge from the capsules upon hatching. During development in the planktonic stage, the lobes become larger and eventually bifurcate
ii) Veligers with larger hatching sizes
Upon hatching, the velar lobes of the larvae are already bifurcated
iii) Veliconchas with larger hatching sizes ( E.g Conus magus)
Veliconchas are usually non-planktonic upon hatching, and have fully formed velar lobes that look similar to those possessed by mature planktonic veliger individuals shortly before they undergo metamorphosis. They also possess a functional foot that allows them to crawl and a calcified shell, features that are characteristic of benthic organisms.
The embryonic development of the Magicina’s Cone (Conus magus)
The egg sizes of the Magician’s cone average between 499-580 μm with the species having nonplanktonic veliconcha larvae, that emerge with fully developed velums.
3.4 Relationship between egg sizes and distribution of Cone snails species
It has been found that widely distributed species tend to be associated with smaller eggs sizes, as the longer planktonic stages allow them to be more well-circulated by ocean currents. On the contrast, species with smaller ranges tend to be associated with species that produce larger eggs(>480 μm), as the larvae hatch at the veliconcha stage and hence tend to be benthic.In Kohn and Perron’s 1994 study, the distribution of the cone snails species across the Phillipine islands and New Caledonia islands were studied.
Phillipine Islands
There have been 160 species of cone snails recorded in the Phillipine Islands, of which 135 were studied. Of these 135 species, 100 were found in shallow waters (<20m) and 69 of these 100 speies, were found to have a wide range that extended to four of the five major lithospheric plates that occurred in the Indopacifc region ( Eurasian Plate, Phillipine Plate, Pacific Plate, Indo-Australian Plate and African plate). All but one of these 69 species had planktonic stages in their developmental trajectory, with the notable exception of the Magician’s cone (Conus magus) which does not have a planktonic stage.
Of the remaining species, 6 of the shallow water species had extremely narrow ranges that did not even extend to the eastern margin of the Pacific plate. There were also 25 othe species that occurred only within 1-3 plates in the area.
New Caledonia
There have been 93 cone snail species recorded in New Caledonia, 85 of which occurs in shallow waters (<20m). A majority (95%) of the species were found to extend to archipelagos in adjacent plates, and 68 of all observed pecies were found to occur on four of all five Indopacific plates( Eurasian Plate, Phillipine Plate, Pacific Plate, Indo-Australian Plate and African plate). All but one of these 68 species are known to have a planktonic larval stage, with again the notable exception of the Magician’s cone (Conus magus).
The notable exception in widely-distributed species: Magician’s Cone (Conus magus)
The Magician’s Cone is known to be one of the most widely distributed cone snail species, and yet has none of the characteristics that are typically associated with widely distributed Cone snail species.
It has a Type I egg mass, which makes the egg capsules less prone to dispersal by ocean currents than the other two types. The Magician’s cone also has a veliconcha hatching stage, meaning it is primarily benthic throughout its life span. Yet, it is interesting to note how it has managed to attain such a wide range of distribution and thus far, there has been no research done to explain this phenomenon.
3.5 Reproductive energetics and evolution of different egg sizes
i) Reproductive energetics in association with growthMost cone snails tend to be an intermediate strategist when it comes to to their growth pattern in relation to reproduction. Female cone snails continue to grow significantly after attaining reproductive size, instead of inhibiting growth and allocating all energy towards reproductive. According Sibily et.al(1985)[21] , growth after reproductive capacity is achieved inherently causes a loss of fitness, as the growth comes at the expense of energy that could be used for reproductive purposes.
However, it can be argued that growth after reproductive capacity has been achieved can increase fitness, if the larger size is associated with increased fecundities, or reduced mortality. While there has been no evidence to suggest that larger sizes reduce mortality for cone snails, it has been shown that larger females do have increased fecundities as they tend to produce a larger clutch size.
ii) Reproductive energetics in association with egg size
It has been found that it is more energetically costly for cone snail species that produce a smaller number of large eggs that have longer pre-hatching developmental periods, compared to cone snail species that produce larger number of smaller eggs that have shorter pre-hatching periods.
Thus far, there have been two hypotheses to explain why certain cone snail species like the Magician’s cone, choose to employ the energetically costly reproductive strategy of producing larger eggs.
1) Size-specific vulnerability: "If the eggs and embryonic stages constitute a ‘safe harbour, embryos suffer significantly lower mortality rates than do free-living juveniles of similar body size, selection will favour evolution of larger egg size."(Shine 1978, 1989)[22]
2) Coevolution of egg size and parental care: " An increase in either variable initiated by some other factor favours an increase in the other "(Nussbaum and Schultz 1989)[23]
6.Taxonomy
6.1. Taxonomic classifcation for Conus magus
| KINGDOM |
ANIMALIA |
| Phylum |
Mollusca |
| Class |
Gastropoda |
| Order |
Sorbeoconcha |
| Family |
Conidae |
| Genus |
Conus |
| Species |
Conus magus |
| Region: Indo-Pacific (Extant) |
| Valid: Yes |
| Type Designation: Kohn, 1963 |
| Type: Neotype |
| Type Repository: UUZM |
| Type Size: 43 x 19 mm |
| Type Locality: None |

Figure 7: Diagram depicting type specimen for Conus magus. Photo Credit: Alan J. Kohn. Retrieved from http://biology.burke.washington.edu/conus/recordview/Conus_magus_327l1l_21111111.html
Synonyms:
· //C. ambaroides// Shikama, 1977
· //C. assimilis// A. Adams, 1854
· //C. borneensis// Sowerby ii, 1866
· //C. caesius// Röding, 1798
· //C. carinatus// Swainson, 1822
· C. cernohorskyi da Motta, 1983
· //C. circae// Sowerby ii, 1858
· //C. consul// Boivin, 1864
· //C. epistomioides// Weinkauff, 1875
· //C. epistomium// Reeve, 1844
· //C. frauenfeldi// Crosse, 1865
· //C. fucatus// Reeve, 1849
· C. fulvobullatus da Motta, 1982
· //C. metcalfii// Reeve, 1843
· //C. raphanus// Hwass in Bruguière, 1792
· //C. rollandi// Bernardi, 1860
· //C. signifer// Crosse, 1865
· //C. tasmaniae// Sowerby ii, 1866
· //C. ustulatus// Reeve, 1844
Subspecies:
//C. decurtata// Dautzenberg, 1910
6.2. Taxonomic uncertainties for Family Conidae
The Conidae together with the Turridae and Terebridae form the Superfamily Conoidea, characterised by the possession of venom gland as well as a radular tooth modified for the purposes of venom injection. As the largest genus of marine invertebrates, it is very challenging to classify cone snails, with new species being discovered annually[25] . At the time of Linneaus, there were 30 known species but presently, there are at least 803 identified species.For the past two centuries, all cone snails have been generally lumped together in the family Conidae based on the characteristic shape of the shell. However, below the family-level, the relationships between the hundreds of cone snails species at the genus and sub-genus level has been highly debated.
The first few attempts at below family-level classification was based on shell characteristics. Some of these divisions were quite vague such as Cotton’s (1945) description of Conidae as one family with 14 groups that “may represent subfamilies” and 29 genera. Walls (1978) divided Conidae into two subfamilies and eight genera[26] and da Motta (1991) classified them as eight genera with 60 subgenera.
A relatively recent novel classification based on radular characteristics was proposed by Tucker & Tenorio (2009)[27] who classified cone snails into five families and 89 genera, with Conidae itself being divided into four subfamilies. However, some authors have argued that radular characteristics make for a poor phylogenetic marker in other conoidean taxa. Additionally, in their study, Tucker & Tenorio (2013) classified the three main lineages identified through molecular phylogenetic sequencing at the family level, and assigned genus-level names to different groups of identified cone snail species. While the criterion for doing such naming was the requirement for monophylyl , with nomenclatural rules followed during the naming process, the threshold at which delimitation occurs at the different ranks is unclear and not objective, since ranks above the species level are often arbitrarily defined and do not have any fixed biological characteristic or property that can define the rank threshold.
To add to the confusion, the concept of what is considered a cone snail itself varies among authors. Some authors(1896) consider Cryptoconidae and Benthofascis to be cone snails, an idea rejected by other authors.
6.3. Classification of the Family Conidae by Molecular Phylogeny
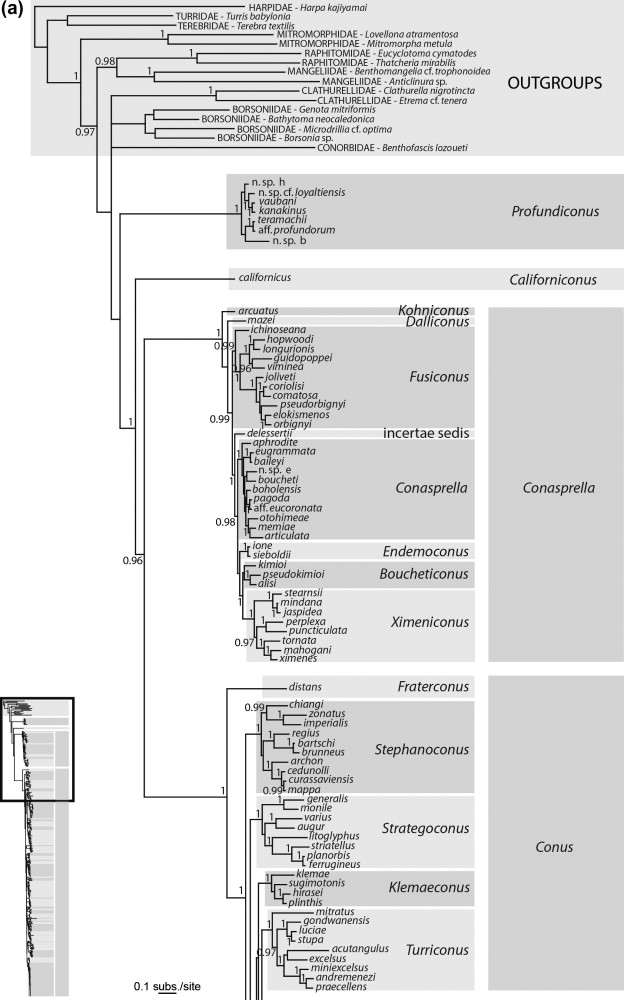
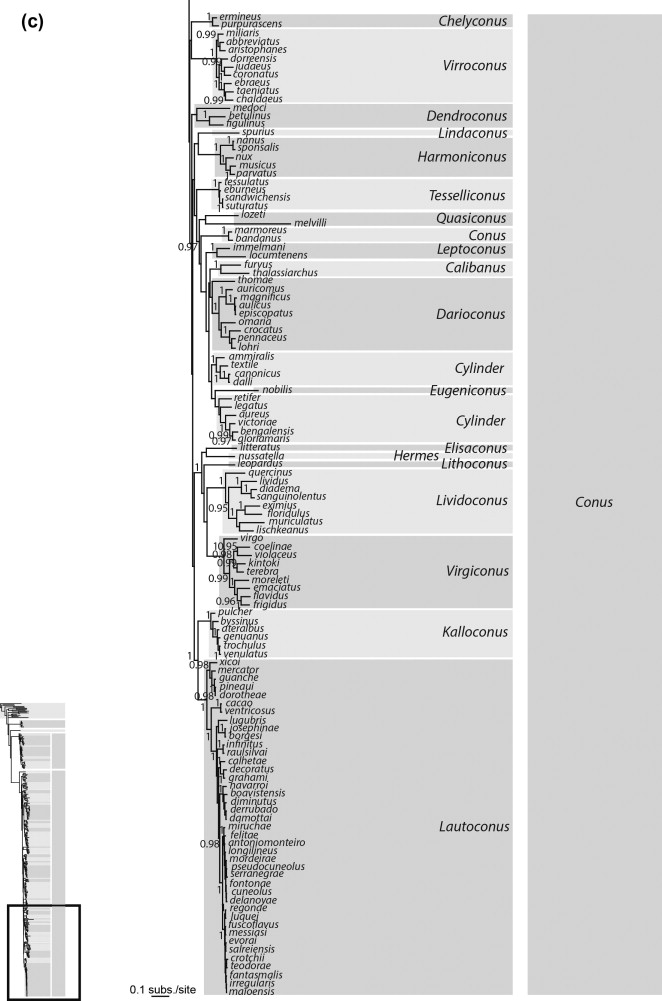
In an attempt to clarify the classification of cone snails at the level below the family, Pulliandre et.al (2014)[28] carried out a molecular phylogenetic analysis based on three mitochondrial genes (CO1, 12S and 16S) sequenced for 320 species. Representatives from the main lineages of Conidae as defined by previous studies were included in the analysis which included Conus californicus, the Small Major Clade and Large Major Clade individuals as described by Duda and Kohn (2005). By Tucker & Tenorio’s (2013) analysis, C.californicus and the Small Major Clade fell under the Family Conilithidae and the Large Major Clade consisted of the family Conidae.The molecular analysis resulted in the following phylogenetic tree shown below.
Figure: The above phylogenetic tree was retrieved from Pulliandre et.al (2014) and displays the classification of cone snails into four distinct clades: C.californicus, Conasprella, Conus and Profundiconus.
Four distinct clades were found in the analysis, with three of them corresponding to the results of previous molecular analysis. The first clade consists of solely the species Conus californicus, the second clade corresponded to the Small Major Clade lineage as defined by Duda and Kohn (2005)[29] and is grouped under Conasprella, the third clade which is the most diverse was the same as the Large Major Clade lineage defined by Duda and Kohn(2005) and is grouped under Conus. However, the fourth clade was described for the first time in this study was found to be somewhat similar to the group Profundiconus as described by Tucker and Tenorio (2009). This clade also included several deep water species that had been included in molecular phylogenetic analysis for the first time, which could partially explain why this clade did not occur in previous molecular phylogenies. Profundiconus has been described a sister group to Conidae but there is a lack of evidence to support this relationship and hence Pulliandre et.al acknowledges that there is insufficient evidence to confirm the inclusion of Profundiconus in Conidae, though morphologically it appears similar to cone snails.
While for 213 of the 320 species analysed, it was found that the DNA analysis produced species delimitations that matched those as defined by shell characters, there were still a high number of species for which the DNA-based species delimitation did not match the morphology-based species delimitation. Pulliandre et.al (2014) acknowledged that this could be due to a few different sources of error.[28]
Firstly, though all the specimens examined had vouchers(or pictures) and were evaluated by several expert taxonomists , the sequences obtained from GenBank did not have voucher material and hence species identification could not be confirmed for those sequences.Secondly, contamination in the sequences could have caused a species misidentification especially in the case where it is the only specimen for a particular species.Thirdly, given that the molecular phylogeny was based on three maternal mitochondrial genes, there is a possibility that the evolutionary history of a particular species is separate from its mitochondrial genome.Lastly, the presence of cryptic species due to the absence of morphological variability between species, or a large range of phenotypic plasticity within a species could have caused incorrect species delimitation. This suggests that taxonomic classification based on morphology for cone snails needs to be improved.
[28]
(Recall the large scale of intra-specific variation for Conus magus as shown in Figure3)
6.4. Species-level identification
In the cases of cryptic species which do not have distinguishable morphological characteristics that can separate them from other species or species with high intra-specific variation such as Conus magus as shown in figure 3, how can species be properly identified?DNA barcoding has been suggested as the most efficient way to confirm species identification especially foe cone snails that hard to identify based on morphological features. There are two described methods for species identification using DNA-barcoding.[10]
The first method involves comparing the DNA barcodes across specimens and setting a threshold to delimit species based on the extent of variation in the sequence. The second method involves building a molecular phylogenetic tree and identifying species as distinctive clades that display monophyly. COI and 16s ribosomal DNA have been proposed as good character-based barcode that can be used for species delimitation for cone snails.
[10]
However, barcodes have their own problems in identifying species such as failing to detect newly diverged species, or further splitting older species since delimitation is based on the number of character differences in the selected genes, which will only accumulate over time and are not truly reflective of evolutionary divergence.
However, due to each cone snail species having its own unique cocktail of conopeptides in its venom ,it has been suggested that species delimitation could potentially be done by screening venom conopeptides. However, due to the millions of unidentified conopeptides across all the cone snail species, employing such venom-based screening system for species delimitation would be extremely difficult. However, it has been found that the genetic variation in conopeptide-encoding venom genes in cone snails is associated with the specificity of their diet, with cone snails with similar diets having similar classes of conopeptides which suggest that venom-based molecular phylogeny would likely allow for classification cone snails at above the species level.
6.5. Which species concept is relevant for cone snails?
Given that we have limited knowledge on the reproductive biology of most cone snail species and it is extremely difficult to observe cone snail reproductive that occurs naturally in the wild, it would be very difficult to apply the Biological and Hennignian species concepts when describing cone snail species since it would be hard to ensure the criterion of naturally occurring reproductive isolation within a species. The evolutionary species concept which defines species as any "entity of organisms" that can maintain its distinct identity over "time and space" and has its "own evolutionary fate and history" that is separate from other such entities can be potentially applied to describe cone snail species. However, due to the vague nature of the evolutionary species concept where the concept of a distinct entity is very subjective and can be applicable to almost anything, this concept has not been used in describing cone snail species.Thus far, it seems that there have been only two relevant species concepts that have been used to describe cone snail species which are the Phylogenetic species concept (sensu Wheeler and Platnick (2000)) and the Phylogenetic species concept (sensu Mishler and Theriot (2000)).[30]
i) Phylogenetic species concept (sensu Wheeler and Platnick (2000))
This concept defines species as lineages that can be diagnosed by a “unique combination of character states” and was the original species concept used to carry out species delimitation for cone snails before molecular techniques were created, when cone snail species were delimited based on differences in shell characteristics. It continues to be used in recent times with Tucker & Tenerio (2009) carrying out species delimitation based on differences in radular morphology characteristics and is also used in the comparison of molecular sequences such as barcodes, where species delimitations occurs due to differences in the character states of the barcode genes.
ii) Phylogenetic species concept (sensu Mishler and Theriot (2000))
This concept defines species as the “least inclusive taxon in a formal phylogenetic classification” that meets the criteria of monophyly and is “deemed worthy of formal recognition”. In the molecular phylogenetic tree constructed by Pulliandre et. al (2014), the main focus was to classify Conidae at the genus and sub-genus level into clades which were monophyletic. Though species-level classification was not the main aim of the analysis, all 320 species analysed in this study were classified into individual monophyletic clades within the three, in acknowledgement that species should meet the criteria of monophyly
[28]
as per the Phylogenetic species concept (sensu Mishler and Theriot (2000)).
Bouchet P and Gofas S. (2015). Conus Linnaeus, 1758. In: MolluscaBase (2016). Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=137813 on 2016-11-24
Tan S K & Woo H P M.2010. A preliminary checklist of the molluscs of Singapore. Raffles Museum of Biodiversity Research
http://www.wildsingapore.com/wildfacts/mollusca/gastropoda/conidae/conidae.htm on 24/11/2016.
Dharma B.2005. Recent and Fossil Indonesian Shells. ConchBooks
Kumar P S, Kumar D S and Umamaheshwari S. 2015. A perspective on toxicology of Conus venom peptides. Asian Pacific Journal of Tropical Medicine:337-351
Olivera B M, Serger J, Horvath M P and Fedosov A E. 2015.Prey-Capture Strategies of Fish-hunting Cone Snails: Behaviour, Neurobiology and Evolution. Brain, Behaviour and Evolution. 86:58-74
Azam L, Dowell C, Watkins M, Stitzel JA, Olivera BM, McIntosh JM. α-Conotoxin BuIA, a novel peptide from Conus bullatus distinguishes among neuronal nicotinic acetylcholine receptors. J Biol Chem. 2005;280:80–7
Dutertre S & Lewis R J. 2013. Cone snail biology, bioprospecting and conservation.Snails: Biology, Ecology and Conservation.Nova Science Publishers. 85-104
Olivera B M, Gray W R, Zeikus R, McIntosh J M, Varga J, Rivier J, De Santos V & Cruz L J.1985.Peptide Neurotoxins from Fish-Hunting Cone Snails.Science.230(4732): 1338-1343
McGivern J.2007.Ziconotide: a review of its pharmacology and use in treatment of pain.Neuropsychiatr Dis Treat. 3(1):69-85
Lewis R J & Garcia M L.2003.Therapeutic potential of venom peptides. Nat Rev Drug Discov. 2. 790-802
Gorson J and Holford M. Small packages, Big Returns: Uncovering the Venom Diversity of Small invertebrate Conoidean snails. Intergrative and Comparative Biology. 56(5): 962-972
Kohn A J and Perron F E.1994. Life History and Biogeography Patterins in Conus.Oxford Biogeography Series No.9. Clarendon Press. Oxford Science Publications.
Siby R, Calow P and Nichols N.1985. Are patterns of growth adaptive? Journal of Theoretical Biology. 112: 553-574
Shine R. 1978. Propagule size and parental care: the ‘safe harbour’ hypothesis. Journal of Theoretical Biology. 75:417-424
Nussbaum R A and Schultz D L. 1989. Coevolution of parental care and egg size. American Naturalist. 122:591-603
Linnaeus, C., 1758. Systema Naturae per Regna Tria Naturae, 10th ed., 1
Rockel D, Korn W & Kohn A J.1995. Manual of the Living Conidae. 1: Grillparzertr.
Walls J G. 1978. Cone Shells a synoposis of the living conidae. T.F.H. Publications.
Tucker J K and Tenorio M J.2009. Systematic classification of Recent and fossil conoidea gastropods. Conchbooks, Hackenheim Germany
Pulliandre N, Bouchet P, Duda JR T F, Kauferstein S, Kohn A J, Olivera B M, Watkins M and Meyer C.2014. Molecular phylogeny and evolution of cone snails ( Gastropoda, Conoidea).78:293-303
Duda T F and A J Kohn. 2005. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol. Phylogenet. Evol. 34: 257–272.
Species Concepts and Phylogenetic theory: A debate. 2000. Ed. Wheeler Q D & Meier R. Columbia University Press